Credit: Institut Laue Langevin
See: https://phys.org/news/2017-02-neutrons-reveal-quantum-tunnelling-graphene.html
and: http://www.cemag.us/news/2017/02/quantum-tunneling-graphene-holds-secret-birth-stars

Credit: Institut Laue Langevin
See: https://phys.org/news/2017-02-neutrons-reveal-quantum-tunnelling-graphene.html
and: http://www.cemag.us/news/2017/02/quantum-tunneling-graphene-holds-secret-birth-stars
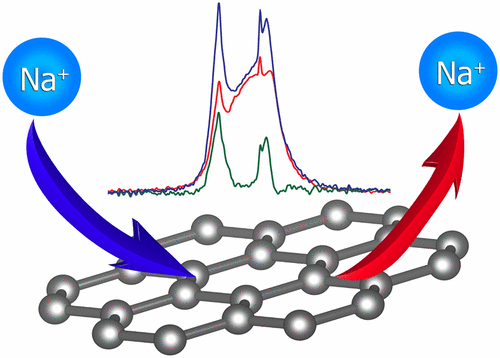
Two chemically synthesized defective graphene materials with distinctly contrasting extended structures and surface chemistry are used to prepare sodium-ion battery electrodes. The difference in electrode performance between the chemically prepared graphene materials is qualified based on correlations with intrinsic structural and chemical dissimilarities. The overall effects of the materials physical and chemical discrepancies are quantified by measuring the electrode capacities after repeated charge/discharge cycles. Solvothermal synthesized graphene (STSG) electrodes produce capacities of 92 mAh/g in sodium-ion batteries after 50 cycles at 10 mA/g, while thermally exfoliated graphite oxide (TEGO) electrodes produce capacities of 248 mAh/g after 50 cycles at 100 mA/g. Solid-state 23Na nuclear magnetic resonance spectroscopy is employed to locally probe distinct sodium environments on and between the surface of the graphene layers after charge/discharge cycles that are responsible for the variations in electrode capacities. Multiple distinct sodium environments of which at least 3 are mobile during the charge–discharge cycle are found in both cases, but the majority of Na is predominantly located in an immobile site, assigned to the solid electrolyte interface (SEI) layer. Mechanisms of sodium insertion and extraction on and between the defective graphene surfaces are proposed and discussed in relation to electrode performance. This work provides a direct account of the chemical and structural environments on the surface of graphene that govern the feasibility of graphene materials for use as sodium-ion battery electrodes.
Reproduced with permission. Copyright 2016, American Chemical Society
This gallery contains 1 photo.
Understanding the mobility of H at the surface of carbon nanostructures is one of the essential ingredients for a deep comprehension of the catalytic formation of H2 in interstellar clouds. We combined neutron vibrational spectroscopy with DFT molecular dynamics simulations to … Continue reading
This gallery contains 1 photo.
The non-equilibrium control of emergent phenomena in solids is an important research frontier, encompassing effects such as the optical enhancement of superconductivity. Nonlinear excitation of certain phonons in bilayer copper oxides was recently shown to induce superconducting-like optical properties at temperatures … Continue reading
This gallery contains 1 photo.
Mixed ionic-electronic conductors (MIEC) are of growing interest for fuel cells, batteries and sensors. By exploiting the unique properties of Li4C60 2D fulleride and poly(ethylene oxide) (PEO), we demonstrated the feasibility of a MIEC where the nature of transport can … Continue reading
This gallery contains 1 photo.
The possibility of producing magnetic graphene nanostructures by functionalization with aromatic radicals has been investigated. Functionalization of graphene basal plane was performed with three types of anilines: 4-bromoaniline, 4-nitroaniline and 4-chloroaniline. The samples were examined by composition analysis with energy-dispersive … Continue reading
This gallery contains 1 photo.
Ammonia has been proposed as an indirect hydrogen carrier, as solid-state ammonia-storage could be easier than directly absorbing hydrogen in materials. Here we investigate the structural evolution of hyper-ammoniated lithium fullerides (ND3)yLi6C60 during ammonia desorption, using in-situ high intensity … Continue reading
This gallery contains 1 photo.
The crystal structure of N,N’-methylenebisacrylamide was determined through the geometry optimization of the molecular unit with density functional theory and conformational analysis, and then through the calculation of the packing via a crystal structure prediction protocol, based on lattice energy … Continue reading
This gallery contains 1 photo.
The performance of graphene, and a few selected derivatives, was investigated as a negative electrode material in sodium and lithium-ion batteries. Hydrogenated graphene shows significant improvement in battery performance compared with as-prepared graphene, with reversible capacities of 488 mAh/g for … Continue reading
This gallery contains 1 photo.
We reported the structural analysis of the highly-doped lithium fulleride Li12C60, performed using low temperature neutron powder diffraction. Although the main reflections could be initially indexed with a fcc cell, Monte Carlo Simulated Annealing suggests an unusual monoclinic arrangement for … Continue reading